Abstract
Rearrangements of the nucleoporin 98 gene (NUP98) define a high-risk subset of acute myeloid leukemia (AML). The resulting fusion oncoproteins (FOs) involve the N-terminal intrinsically disordered region of NUP98 and the C-terminal portion of one of more than thirty fusion partners. Approximately one-third of fusion partners have DNA-binding homeodomains, and the remainder frequently contain other domains involved in gene regulation, in particular chromatin remodeling. We and others recently showed that NUP98 FOs localize in nuclear puncta and undergo liquid-liquid phase separation (LLPS), which is required for cell transformation and gene expression changes associated with NUP98 rearrangements (Cancer Discov 2022;12:1152, Nature 2021;595:591, Nat Struct Mol Biol 2021;28:190). However, which proteins interact with NUP98 FOs in nuclear puncta, how they influence leukemogenic gene expression, and functional differences between fusion partners are incompletely understood.
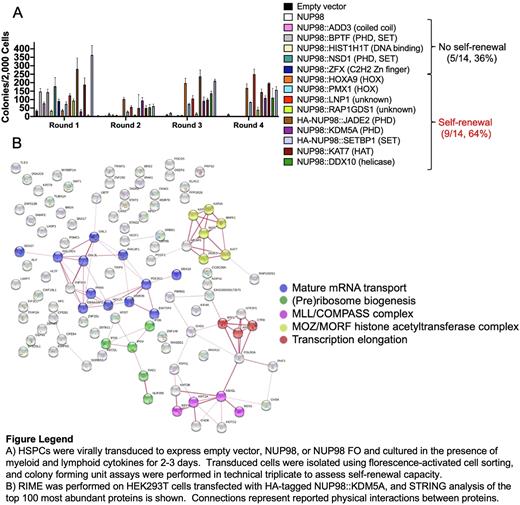
We first tested the ability of fourteen representative NUP98 FOs to confer self-renewal using mouse HSPC colony forming unit assays, demonstrating that nine (64%) FOs were transforming (Fig A). Further, gene expression studies of ex vivo NUP98 FO-expressing cells identified transcriptional targets both shared by several fusions (including Hoxa genes, Nkx2-3, Gata3, and Mpl) and unique to individual fusions. We next performed rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) to explore the protein interactions of five fusions (NUP98::HOXA9, ::KDM5A, ::NSD1, ::LNP1, and ::PMX1) in transfected HEK293T cells and ascertain the composition of NUP98 FO-associated puncta (Fig B). RIME enriches for protein-protein interactions that occur on chromatin and may identify FO interactions required for NUP98 FO gene deregulation. Interactions with proteins involved in mRNA transport (including RAE1 and XPO1) and in (pre)ribosomal biogenesis were shared across multiple NUP98 FOs. Notably, bromodomain-containing proteins were among the significant interactions for NUP98::KDM5A and NUP98::NSD1, a result consistent with previous work from our group showing that NUP98-rearranged cells are sensitive to BRD4 inhibition (Blood 2021;137:1628). RIME also showed that members of the MLL/COMPASS complex (including KMT2A and Menin) interact with NUP98::KDM5A and NUP98::NSD1, and pharmacologic inhibition of this complex has also been reported as an effective therapeutic strategy in NUP98-rearranged AML (Blood 2022;139:894). Finally, gene set enrichment analysis of protein interactions for NUP98::KDM5A and NUP98::NSD1 ranked by abundance highlighted proteins involved in histone H3 acetylation, including several members of the MOZ/MORF histone acetyltransferase (HAT) complexes (including MOZ, MORF, and BRPF1).
To investigate the contribution of chromatin remodeling complexes including HATs to NUP98 FO-driven leukemogenesis, we performed an in vivo CRISPR screen with a library of epigenetic gRNAs in cells transduced to express NUP98::KDM5A. Our results suggested that BRPF1, an epigenetic writer that exists in complex with MOZ, MORF, and/or HBO1, is a molecular dependency in NUP98::KDM5A AML. To validate this finding, we performed CRISPR gene editing in Nup98::Kdm5a;Vav-Cre Cas9+ HSPCs to inactivate 11 genes, including Brpf1 and members of BRPF1-containing HAT complexes. Competitive co-culture of control and HAT complex gene-targeted cells showed that inactivation of Brpf1, Moz, Hbo1, Brd1 and Meaf6 decreased cell fitness, with no effect following inactivation of the HAT complex members Morf, Ing5, Jade1/2/3 or Brpf3. Finally, a counter screen in wildtype cells showed that many of the HAT complex genes are not essential in normal HSPCs, suggesting that lysine acetyltransferase complexes have a unique and potentially targetable role in NUP98::KDM5A FO-expressing cells.
In summary, our studies detail the overlapping and distinct members of the protein interactome for multiple NUP98 FOs and demonstrate that MOZ and HBO1 complexes play an important role in the fitness of cells expressing NUP98::KDM5A.
Disclosures
Iacobucci:Mission Bio: Honoraria. Mullighan:Abbvie: Research Funding; FAZE: Honoraria; Pfizer: Research Funding; Illumina: Honoraria; Amgen: Honoraria; BEAM: Honoraria; Consulting: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal